Significance Statement
Hydrogen has been discussed as a clean energy carrier, when generated from non-carbonaceous, regenerative energy sources. Among various hydrogen technologies such as hydrogen generation (water electrolysis or hydrocarbons reforming), hydrogen processing (purification) or hydrogen conversion (electricity and heat in fuel cells), hydrogen storage makes an important contribution towards realisation of hydrogen based economy.
German researchers developed an aluminium alloy tank for the chemical storage of hydrogen using almost 2 kg of solid complex aluminium hydride Na3AlH6 doped with TiCl3 and activated carbon. The authors have stored 38 g of hydrogen in the 3 l aluminium alloy tank. Their work is now published in peer-reviewed Journal of Power Sources.
The advantages of storing hydrogen in solid state metal hydride Na3AlH6 are high volumetric storage capacity with a theoretical maximum of 253 g dm-3, which is 3.6 times the density of liquid hydrogen, lower costs for refueling of hydrogen and the possibility to thermally couple the storage tank with fuel cells.
The storage material was prepared by mechanical milling under an inert atmosphere using a planetary ball mill. The team of authors has filled this almost 2 kg of hydrogen storage material in 18 fills into the tank. After pouring of some amount of hydride the tank was placed on a densification apparatus to ensure that the material would be evenly distributed and densified. Multiple taps with the densification apparatus were recommended to provide reliable determination of tapped density of 620 g dm-3.
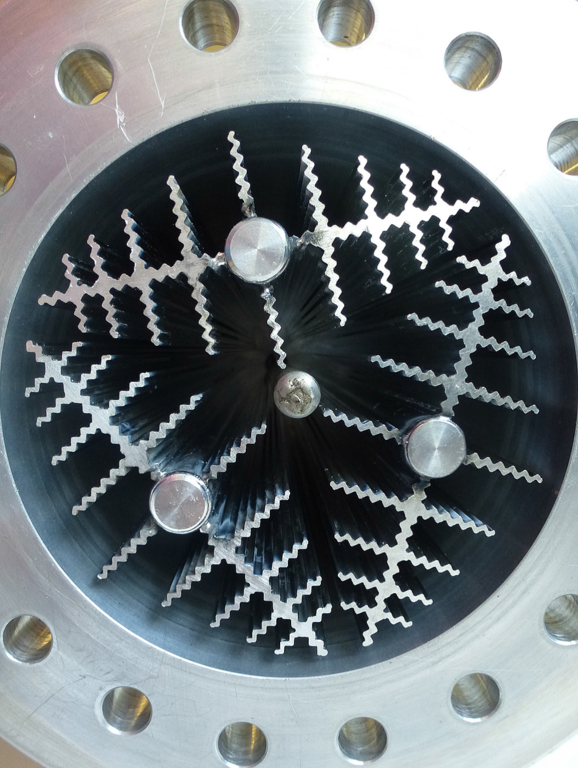
The hydrogen storage tank and the heat exchangers were fabricated from an aluminium-magnesium-silicon based alloy. The tank and the heat exchangers were manufactured in an extrusion moulding process, which can enable a serial production of lightweight tanks for solid state hydrogen storage. The corrugated heat exchangers are optimized for equal heat distribution.
After filling the tank, it was heated under hydrogen pressure. In hydrogenation process, when the hydrogenation pressure of 2.5 MPa was applied and a temperature of 38 °C was reached, the decomposed storage materials (sodium hydride and aluminium) started to react with hydrogen to form Na3AlH6. The absorption of hydrogen increased with rising temperature. The highest hydrogen flow reached 1022 dm3 h-1 whereas the temperature of the storage material rose to 123 °C. 45 min after the start, the flow of hydrogen began to decrease, which means that hydrogenation process came to an end.
Dehydrogenation process of Na3AlH6 was carried out when the temperature increased up to 180 °C. Hydrogen flow was kept constant at 220 dm3 h-1. After 100 min the hydrogen pressure dropped from initial value of 1.64 MPa to 0.1 MPa. In this test around 38 g of hydrogen, which corresponds to a gravimetric capacity of 2.0 mass %, was evolved. Hence the tank was subjected to 31 tests in which 16 dehydrogenation and 15 hydrogenation tests were performed without any failure.
The aim of this project was to demonstrate the operation of the lightweight aluminum alloy tank using a solid complex aluminium hydride for storing hydrogen. As a result, possible implementations of such a tank operating at lower hydrogen pressure in combination with a high-temperature fuel cell system in stationary application can be safer compared to systems working under much higher hydrogen pressure.
Reference
Urbanczyk, R.1, Peinecke, K.2, Meggouh, M.2, Minne, P.3, Peil, S.1, Bathen, D.3, Felderhoff, M.2, Design and operation of an aluminium alloy tank using doped Na3AlH6 in kg scale for hydrogen storage. Journal of Power Sources, Volume 324, 2016, Pages 589–597.
[expand title=”Show Affiliations”]- Institut für Energie- und Umwelttechnik e.V., Bliersheimer Str. 58-60, 47229 Duisburg, Germany.
- Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr, Germany.
- Universität Duisburg-Essen, Lehrstuhl für Thermische Verfahrenstechnik, Lotharstraße 1, 47057 Duisburg, Germany.
Go To Journal of Power Sources
 Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.
Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.







