Significance Statement
NH3 is the most common substitute in cooling systems, and it is a toxic compound and volatile, which is very harmful to the human body. On the other hand, NH3 is a promising candidate as a future energy carrier in vehicles and as an NH3 reservoir for the selective catalytic reduction (SCR) of NOx gases in diesel cars and trucks. Thus, it is highly desirable to find a effective method to sensor and capture the NH3 gases for atmospheric environmental controls and industry applications. Very recently, MXene has attracted worldwide attention due to its potential applications on electrochemical energy storage. MXene is found to be terminated with several kinds of functional group (such as F, OH and/or O), with only Sc2CX2 (X = F, OH, and O), Ti2CO2, Zr2CO2, Hf2CO2 exhibiting semiconductor properties.
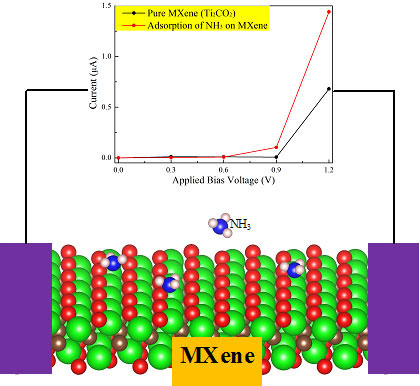
In a series of works, we first studied the potential of MXene as the NH3 sensor and capturer by first-principle simulation. The preference adsorption of NH3 against the other gas molecules (H2, CH4, CO, CO2, N2, NO2 and O2) on MXene suggests the selectivity of MXene towards NH3. The distinct transport feature with a dramatic change of current-voltage (I-V) relation before and after NH3 adsorption indicates the strong sensitivity of MXene towards NH3. More importantly, the adsorption of NH3 on MXene could be further strengthened with the increase of applied strain on MXene, while the adsorption of other gases on MXene is still weak under the same strain, indicating that the capture of NH3 on MXene under the strain is highly preferred over other gas molecules.
However, one of the main challenge for an optimal NH3 sensor or capture is to realize the facile release of NH3 from substrates, which is due to the tendency of NH3 strong interaction with many substrates (including MXenes). To overcome this problem, we subsequently studied the interaction between NH3 and MXenes with different charge states by using first-principles simulations. The results show that the interaction strength between NH3 and MXenes can be significantly weakened by introducing electrons to the adsorbent, finally leading to the efficient release of NH3 gas from these materials.
Our study widens the application of MXene not only as the battery material, but also as the reusable material for NH3 sensor or capturer by controlling the strain or charge injection.
Journal Reference
Sensors and Actuators B: Chemical, Volume 235, 1 November 2016, Pages 103–109
Xue-fang Yu1, Yan-chun Li2, Jian-bo Cheng1, Zhen-bo Liu1, Qing-zhong Li1, Wen-zuo Li1, Xin Yang1, Bo Xiao*1
[expand title=”Show Affiliations”]- The Laboratory of Theoretical and Computational Chemistry, School of Chemistry and Chemical Engineering,Yantai University, Yantai 264005, China
- Institute of Theoretical Chemistry,Jilin University, Changchun 130021, China
Abstract
NH3 is one of the most common resources in chemical industry, which is very harmful to the human body. Thus, it becomes highly desirable to design advanced materials for efficient NH3 sensor or capturer. However, one of the main challenge is the facile release of NH3 gas from substrates due to the tendency of NH3 strong interaction with many substrates. In this work, the interaction between NH3 and O-terminated semiconducting MXenes (M2CO2, M = Sc, Ti, Zr, and Hf) with different charge states is considered by using first-principles simulations. Our results reveal that the NH3 could be strongly adsorbed on M2CO2 with apparent charge transfer, which renders them the potential candidates as the NH3 sensor or capturer. In particular, the process of NH3 release could be simply realized by tuning the electrons injected into M2CO2. Taking Zr2CO2 as an example, it is highly selective towards NH3 against other common gas molecules, and the adsorption energy dramatically increases from −0.81 eV to −0.20 eV when extra two electrons are injected into the 3 × 3 Zr2CO2 sheet. Our study demonstrates that O-terminated semiconducting MXenes are excellent materials for NH3 sensor or capturer, with highly reversible release and capture by controlling the charge states on the system.
Go To Sensors and Actuators B: ChemicalAdditional References:
 Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.
Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.



