Significance Statement
Currently the safest and most effective method to store hydrogen is by using solid media, such as sorbent materials and hydrides. The insufficient value of the specific capacity indexes as well as kinetic and thermodynamic parameters for reversible hydrogen storage has hindered its practical use. The US Department of Energy in conjunction with the automotive industry defined the criteria for an onboard hydrogen storage system.
Professor Nikolay Galushkin and colleagues from Don State Technical University in Russia proposed a thermal runaway as a new high-performance method of releasing hydrogen from hydrides. The research is now published in peer-reviewed journal, International Journal of Hydrogen Energy.
There are two methods of releasing hydrogen from hydrides, thermo-chemical and chemical methods. For chemical method regeneration of initial hydrides for the reaction is principally possible but inexpedient for economic reasons. This is why, this method is considered unacceptable for hydrogen storage systems. Thermo-chemical method is currently the main method of releasing hydrogen from the hydrides. As for the thermo-chemical method, a process of hydrogenation/dehydrogenation is determined by setting of certain values of hydrogen’s pressure and temperature. However, the parameters obtained in the thermo-chemical method for all known hydrides are far from the requirements of the US Department of Energy.
In view of overcoming this shortcoming, the authors introduced the thermal runaway as a principally new method of hydrogen desorption from hydrides. Their work was based on kinetic and thermodynamic parameters of thermal runaway as a new method of hydrogen desorption and also compare the obtained parameters with correspondent parameters obtained with thermo-chemical method and with US Department of Energy requirements.
In their study, the KSX-25 batteries with sintered electrodes by capacity 25 Ah were used, which with a service life of more than five years, contains approximately 800 liters of hydrogen (or 20.1 wt% of hydrogen, for nickel matrix of oxide-nickel electrode). The hydrogen desorption rate was determined using the data within the scale of 20-80% completion desorption processes. They evaluated the kinetic and thermodynamic parameters of oxide-nickel and cadmium electrodes as hydrogen storage using the thermo-chemical and thermal runaway methods of dehydrogenation.
The authors established a number of advantages of thermal runaway compared to traditional thermo-chemical method.
Firstly, on its kinetic and thermodynamic parameters, the thermal runaway method is far superior to the criteria of US Department of Energy as well as to the best values obtained in the frame of the traditional thermo-chemical method. For its work, it does not require any certain values of temperature or pressure; it can work at any ambient temperature and pressure. For its work (unlike the traditional thermo-chemical method), the thermal runaway method does not require any additional energy expenses. On the contrary, in a case of use of the hydrogen desorption on the thermal runaway method, a lot of energy is released, which can be used profitably.
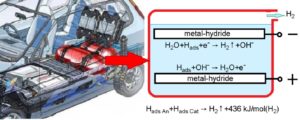
Secondly, the hydrogen desorption on the thermal runaway method runs with aid of electrochemical reactions. This is why, this process is easily controllable by electrotechnical methods and hence, it is much less inertial than the thermal processes used in the traditional method.
Thirdly, the thermal runway method proposed in this study was very efficient in releasing hydrogen from any metal-hydrides and this serve as advantage in hydrogen storage systems.
Journal Reference
N.E. Galushkin, N. N. Yazvinskaya, and D .N. Galushkin, Thermal Runaway as a New High-Performance Method of Desorption of Hydrogen from Hydrides, International journal of hydrogen energy 41 (2016) 14813-14819.
Don State Technical University, Laboratory of Electrochemical and Hydrogen Energy, 147 Shevchenko Street, Town of Shakhty, Rostov Region, 346500, Russia.
Go To International Journal of Hydrogen Energy
 Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.
Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.




