Significance Statement
Hydrogen fuel is a promising option in providing a cleaner energy that is less dependent on fossil fuel. Isolating hydrogen from water which is cleaner and sustainable is commonly performed by electrocatalysis or photoelectrocatalysis. They however suffer from poor catalyst stability and slow kinetics of hydrogen release at the cathode. An example of an oxygen evolution reaction catalyst includes ruthenium (IV) oxide which deactivates at high potential by formation at higher valence ruthenium oxides. This has led to extensive research on abundant, inexpensive, durable and active alternative electrocatalyst for the oxygen evolution reaction.
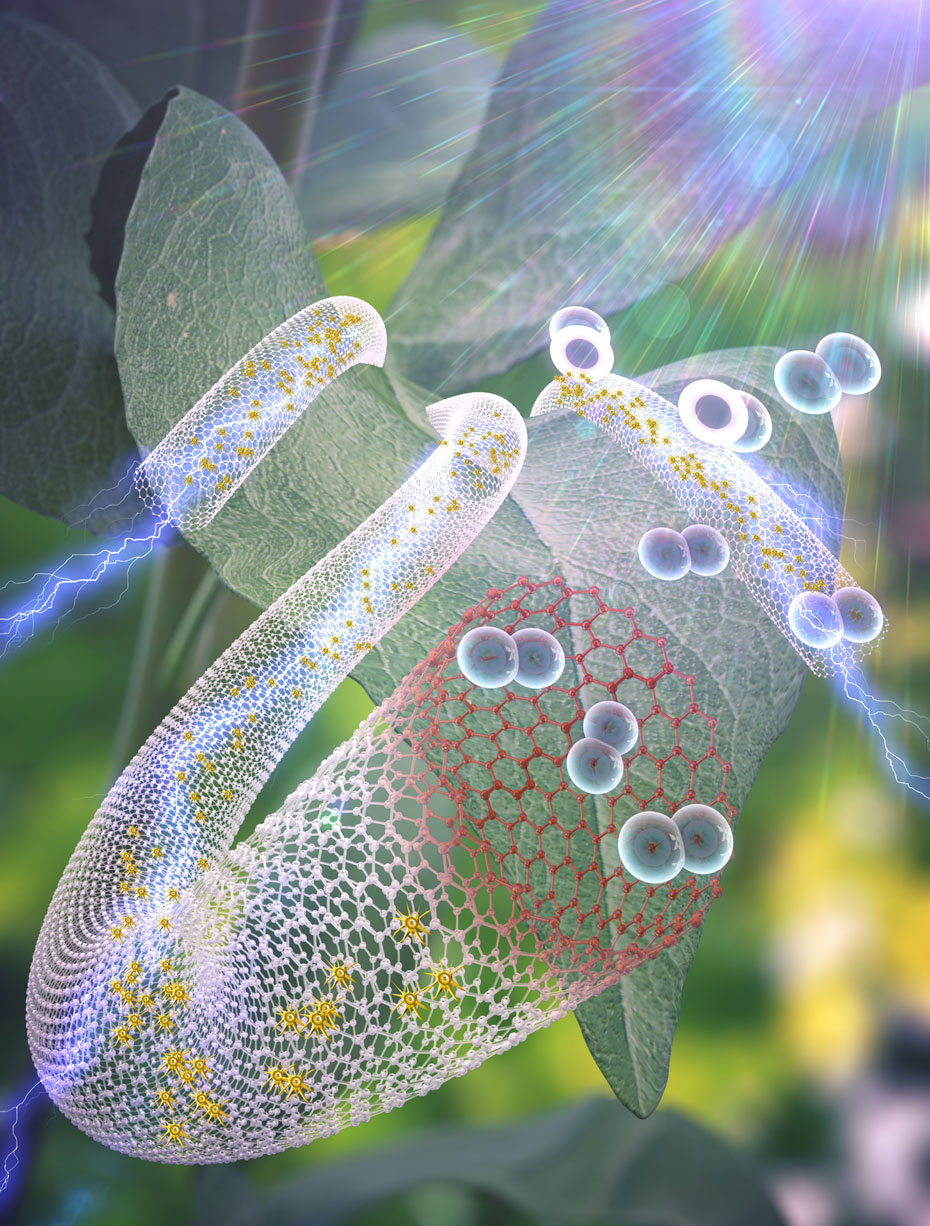
Researchers led by Professor Steven L. Suib from the University of Connecticut and their collaborators at Brookhaven National Laboratory developed a sequential two-step or bi-doping strategy as a novel route to control the active sites of sulfur-doped multi-wall carbon nanotubes at the atomic scale. The research work is now published in Advanced Energy Materials.
For cost reduction, non-noble metal oxides including perovskites and spinels such as Co3O4, LiCoO2 and ZnxCo3-xO4 have been used. Research has shown that the presence of amorphous surfaces in metal oxides, method of oxidation, crystallinity, and pH range of reaction play a major role in oxidation evolution reaction activities. The nature of binding and doping-induced defects are very critical to enhance the activity and stability of metal-free catalysts for oxygen evolution reactions.
However, few reports about metal-free electrocatalysts for oxygen evolution reaction exist due to their low activities and stabilities likely due to the nature of binding between the heteroatom and carbon skeleton.
For synthesis of sulfur-doped carbon nanotubes, partially unzipped carbon nanotubes (Ox-CNT) was prepared by the same procedure reported by Kosynkin et al. (2009) with little modification. Doping of sulfur was done by sonification of Ox-CNT (300 mg) into 30 mL double deionized water and then thiourea (3 g) was added into the solution with stirring (S-CNT180°C). The second doping of sulfur (S,S’-CNT1000°C) was produced from sonification of S-CNT180°C (100 mg) in 10 mL ethanol along with BDS(500 mg). S-CNT1000°C was produced from sonification of S-CNT180°C at 10000C for 1 h. S’-CNT1000°C was obtained by using the same conditions of synthesis S,S’-CNT1000°C. The overall synthesis was achieved by using the incipient wetness impregnation method.
Active sites of sulfur-doped carbon nanotubes were characterized by high-resolution transmission electron microscopy (HRTEM), X-ray photoelectron spectroscopy (XPS), Cs-aberration-corrected scanning transmission electron microscopy, high angle annular dark-field imaging, and STEM electron energy loss spectroscopy mapping and spectra. The nature of the environment of sulfur active sites was examined using fluorescence emission of sulfur K-edge by X-ray adsorption near edge spectroscopy.
High-resolution transmission electron microscopy imaging show carbon nanotubes approximately 27 nm in diameter with fringes parallel with slight undulation. Although a few bifurcate, all are well-ordered to the margin of carbon nanotubes. Upon hydrothermal treatment with thiourea (S-CNT1000°C), a disordered surface zone of 0.4-1.5 nm in width of 2-4 fringes is formed in which the definition of fringes is lost. With the second step of doping with benzyl disulfide (S,S’-CNT1000°C), the zone of disorder is removed and fringes while controlled extend to the carbon nanotube margin, but do not everywhere lie parallel to this margin.
HAADF-STEM images and STEM-EELS confirmed that sulfur heteroatoms have a uniform distribution with carbon nanotubes and nanolobes representing partially unzipped carbon nanotubes which makes active cells more accessible to the reaction medium.
Cyclic voltammetry of S,S’-CNT1000°C revealed the absence of any capacitance which was also supported by increasing the scan rate of linear sweep voltammetry from 5 to 200 mVs-1 without changing current density. The catalytic activity of S,S’-CNT1000°C showed an overpotential of 10 mAcm-2 which is required for solar operation at 350 mV.
The El-Sawy et al. (2016) method of doping increased the oxygen evolution reaction performance of carbon nanotubes by boosting catalytic activity with an over potential of 350 mV at a current density of 10 mAcm-2 and resulted in an enhanced turnover frequency of two orders of magnitude greater than that of state-of-the-art catalysts for the oxygen evolution reaction.
Journal Reference
Abdelhamid M. El-Sawy1,2,3, Islam M. Mosa1,3,Dong Su 4, Curtis J. Guild 1, Syed Khalid1, Raymond Joesten 1, James F. Rusling1,2, Steven L. Suib1,2,5. Controlling the Active Sites of Sulfur-Doped Carbon Nanotube-Graphene Nanolobes for Highly Efficient Oxygen Evolution and Reduction Catalysis. Advanced Energy Materials , Vol 6 Issue 5,2016, 1501966.
[expand title=”Show Affiliations”]- Department of Chemistry, University of Connecticut, Storrs, CT, USA
- Institute of Materials Science, University of Connecticut, Storrs, CT, USA
- Department of Chemistry, Faculty of Science, Tanta University, Tanta, Egypt
- Center for Functional Nanomaterials, Brookhaven National Laboratory, Upton, NY, USA
- Chemical and Biomolecular Engineering, University of Connecticut, Storrs, CT, USA
Go To Advanced Energy Materials
 Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.
Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.









