Significance
Glaucoma is a multifactorial optic degenerative neuropathy characterized by the loss of retinal ganglion cells. Glaucoma poses a significant public health concern as it is the second leading cause of blindness after cataracts, and this blindness is usually irreversible. Dr. Pantcheva, a glaucoma expert, highlights the clinical impact: “It is expected that approximately 76 million people suffer from glaucoma with that number estimated to reach over 112 million people in the next two decades. Although the pathogenesis of glaucoma is not known, there are many well-known risk factors, such as elevated intraocular pressure (IOP), that could lead to irreversible blindness if not addressed in time.”
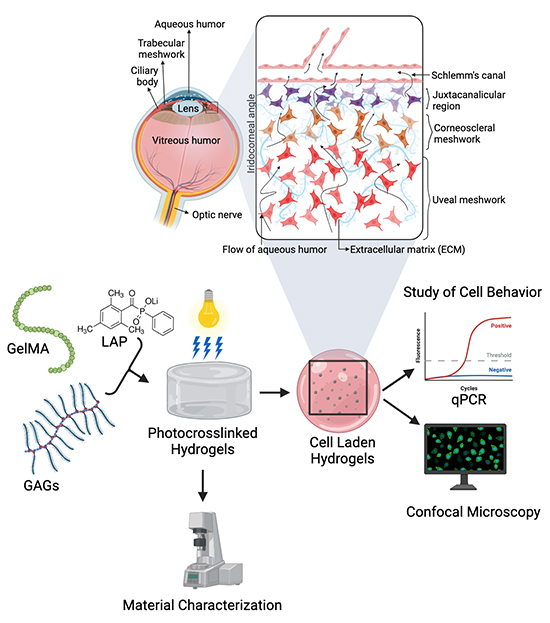
Primary open-angle glaucoma (POAG) is the most recognized and most common type of glaucoma, representing ~ 70 of all cases. Patients suffering from POAG experience increased IOP due to the accumulation of the aqueous humor within the eye. The rise in IOP is attributed to the imbalance between the production and drainage of the aqueous humor through the trabecular meshwork, a porous tissue composed of glycosaminoglycans (GAGs) and collagen. The trabecular meshwork (TM) consists of three main regions responsible for filtering the aqueous humor: uveal meshwork, juxtacanalicular tissue and corneoscleral meshwork. Although the aqueous humor production rate is usually normal in most POAG cases, efficient aqueous humor drainage is affected by the buildup of extracellular matrix (ECM) components within the TM tissue.
Despite the lack of a known cure for glaucoma, available treatment options mainly involve applying drugs via eye drops and sometimes surgery to lower IOP by increasing the drainage of aqueous humor or reducing its production rate. Recent research has shown that the behaviors of trabecular meshwork cells are mainly affected by their microenvironmental conditions, and studies carried out on 2D plastic substrates fail to accurately reflect the behaviors of the TM cells in their native settings. Three-dimensional culture models aim to mimic the proper interactions of both cell–cell and cell–environment providing for the complex biochemical and physical signals as found in in vivo tissue structure. Therefore, developing new effective glaucoma therapeutics requires a thorough understanding of the human TM (hTM) cell behaviors and their associated response in different biological interactions within the 3D complexity of the native trabecular meshwork.
Herein, a team of researchers from Colorado School of Mines: Mr. Bikram Adhikari, Mr. Benjamin Stinson, Dr. Matthew Osmond and Professor Melissa Krebs in collaboration with Dr. Mina Pantcheva from the University of Colorado School of Medicine fabricated a 3D hydrogel-based scaffold model to examine the effects of extracellular environment on TM cells in a biomimetic 3D hydrogel microenvironment. The work is currently published in the journal, Industrial & Engineering Chemistry Research.
In their approach, the 3D model consisted of photoinduced gelatin-methacrylate (GelMA) hydrogels capable of mimicking the native environment of the trabecular meshwork cells. The composition of the GelMA scaffolds was varied by adding the GAGs chondroitin sulfate and/or hyaluronic acid to increase its 3D complexity. The concentration of the GelMA used was optimized by characterizing the hydrogels for mechanical behaviors like viscosity and storage moduli data. The hTM cells were cultured on GelMA scaffolds and the effects of dexamethasone (a steroid known to impact TM cells) and GAG on cell behaviors were examined.
The authors’ findings showed that the GAG composition and GelMA concentration affected the fibronectin expression and proliferation of the hTM cells in the presence and absence of dexamethasone. Fibronectin plays a vital role in holding other proteins within the trabecular meshwork ECM, which plays a fundamental role in regulating IOP in normal and glaucomatous eyes. ECM remodeling is likely responsible for influencing the elevated IOP in glaucoma. Furthermore, the substrate-driven change in the mechanical properties of cell proliferation and fibronectin expression provided more insights into the behaviors of trabecular meshwork cells and their associated interactions with the ECM, thereby providing a better understanding of aqueous humor drainage dynamics.
In a nutshell, significant advances in our understanding of the normal function of the trabecular meshwork cells have come from pioneering studies on cultured trabecular meshwork cells from several species. Colorado School of Mines scientists are the first research group to successfully use photo-cross-linked GelMA hydrogels to model successfully the trabecular meshwork in a biomimetic 3D microenvironment. The findings provided a better illustration of the behavior of trabecular meshwork cells, including the proliferation and expression of ECM proteins, when cultured on different GelMA scaffolds. In a statement to Advances in Engineering, Professor Melissa Krebs, the lead corresponding author, stated that “the new model platform will allow us in the future to check the effectiveness of therapeutic compounds in counteracting the oxidative and/or pressure damage during glaucoma evolution.”
Reference
Adhikari, B., Stinson, B., Osmond, M., Pantcheva, M., & Krebs, M. (2021). Photoinduced Gelatin-Methacrylate Scaffolds to Examine the Impact of Extracellular Environment on Trabecular Meshwork Cells. Industrial & Engineering Chemistry Research, 60(48), 17417-17428.
 Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.
Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.


