Significance
Polycyclic aromatic hydrocarbons are generally formed from incomplete combustion of organic materials. They contain compounds such as benzene rings that have negative impacts on both humans and the environment. Past research studies have attempted to improve their formation which may reduce their effects in one way or the other. Particularly, kinetic mechanism developed for hydrocarbon fuel combustion has been used in predicting formation of aromatic hydrocarbons. Subsequent development has been undertaken to improve this method for application in wide operating conditions. Researchers have, however, revealed that these mechanisms are incapable of predicting the temperature effects on the formation of polycyclic aromatic hydrocarbons in pyrolysis of acetylene, an intermediate product that is generally available during the combustion of hydrocarbons. To this end, continuous refining of the reaction pathways of acetylene pyrolysis is highly desirable.
In a recent research published in the Fuel journal, a group of researchers: Dr. Hairong Tao from Beijing Normal University, Hsin-Yao Wang (MS Student) from National Sun Yat-Sen University, Professor Wei Ren from the Chinese University of Hong Kong and Professor Kuang Lin from National Tsing Hua University investigated the effects of temperature on the formation of polycyclic aromatic hydrocarbons in pyrolysis of acetylene using a newly proposed kinetic mechanism. The main objective was to predict the yield of species concentrations measured in a tubular flow reactor especially in cases involving the formation of quasi-isothermal zones.
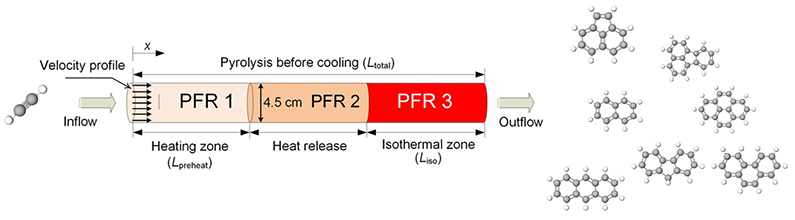
It is worth noting that the existing kinetic mechanisms of C2H2- polycyclic aromatic hydrocarbons are less accurate and unsuitable for predicting C2H2 conversion rate as well as the formation of two- to four-ring aromatics. Unlike the previous computational models for tubular flow reactors, the proposed reactor module comprised of three serially connected plug flow reactors taking into consideration the three zones: preheating, heat release and isothermal conditions. The plug flow reactors were particularly used to represent the characteristics of thermalization effects at chemically reacting conditions. Furthermore, the presented mechanism is generally a combination of the kinetic databases derived from two existing mechanisms but with added missing reactions and rate constants.
The authors observed that the reactor configuration was capable of accurately predicting the complex chemical kinetics involved in the formation of light-weight polycyclic aromatic hydrocarbons in the pyrolysis of acetylene at different temperatures. For instance, both the refined kinetic mechanism and the newly adopted rector module recorded an improvement in the prediction of C2H2 conversion and polycyclic aromatic hydrocarbons formation during the pyrolysis of acetylene at a temperature range of 970-1360k and a pressure of 1 atm. The effectiveness of the reactor was validated by comparing the present calculations of fuel conversion and hydrogen formation values obtained from the same mechanism to the previous ones. The computed results of the present mechanism made up of 290 species and 1175 reactions agreed well with the experimental values. Furthermore, the reaction pathway analysis was performed at different temperatures to identify the reaction pathways altered by the temperature change.
In summary, the study insights highlight the critical role of the reaction pathways in governing the temperature-dependent formation of lightweight polycyclic aromatic hydrocarbons from the pyrolysis of acetylene. Based on the results, the mechanism offers a relatively high predictive capability that can be potentially used as the base model in oxidation mechanisms of hydrocarbon fuels, as per the statement from Professor Kuang Lin, the lead and corresponding author to Advances in Engineering.

Reference
Tao, H., Wang, H., Ren, W., & Lin, K. (2019). Kinetic mechanism for modeling the temperature effect on PAH formation in pyrolysis of acetylene. Fuel, 255, 115796.
 Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.
Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.


