Significance Statement
The vast majority of raw materials used nowadays in chemical industries come from fossil fuels and only about 10% of the feedstock can be traced to renewable resources. In view of the dwindling fossil fuel resources as well as the ever-rising need for sustainability, chemical industries and academia have grown interest in the development of fuels, materials, and chemicals from renewable resources. For this reason, to realize a more sustainable economy, the shift from petro-refinery to bio-refinery is of utmost importance.
To be more precise, the transformation of vegetable oils, reference to their low cost, large-scale availability, and biodegradability, has received renewed research attention. Above their large-scale availability, an array of products can be obtained from seed oils making them good fit for biorefinery as environmentally friendly alternative. For the conversion as well as formation of new carbon-carbon double bonds, olefin metathesis is a versatile synthetic formation tool, which has been applied for both pure and applied chemistry.
Olefin metathesis reactions have been classified into cross-metathesis, ring opening and ring closing. Cross-metathesis is becoming a standard approach with a number of industrial uses such as Shell higher olefin process. Unfortunately, the selectivity of ruthenium complexes for the synthesis of α-olefins from renewable seed oils has been identified as poor. A number of research works have focused on the conversion of plant-oil-derived fatty acids into useful products using ethenolysis chemistry.
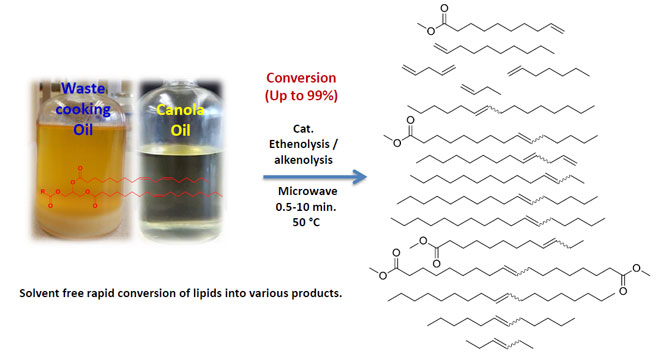
Irrespective of the considerable improvements in the metathesis, there is still the desire for solvent-free rapid conversion of plant oils into preferred terminal olefins for the effective transformation of plant oils into downstream products. Aman Ullah and Muhammad Arshad at the University of Alberta developed a microwave assisted ethenolysis as well as alkenolysis technique by applying ethylene as well as a diolefin under microwave irradiation conditions. This was aimed at rapid and efficient conversion of canola oil along with mixtures of canola methyl esters as well as recycled cooking oil into linear α-olefins under solvent-free metathesis conditions. Their research work is published in ChemSusChem
The application of the microwave electromagnetic radiation for the cross-metathesis of vegetable oils and fatty esters of vegetable oils with 1,5-hexadiene and ethylene led to an increase in conversion rates, turnover numbers and turn-over frequencies in a short reaction duration under solvent-free conditions and low catalysts concentrations. Ethenolysis of canola oil methyl esters and recycled cooking oil provided better results. Out of the four catalysts, Hoveyda–Grubbs catalyst 2nd generation was identified to be the most effective at 50 °C, providing effective conversion with better yield as well as selectivity in a majority of cases for ethenolysis and alkenolysis reactions.
The authors achieved high turnover frequency values of 19110/min for direct ethenolysis of canola oil, 10900/min for the cross metathesis of 1,5-hexadiene, 21450/min for ethenolysis of canola methyl esters, and 15840/min for ethenolysis of recycled cooking oil methyl esters. The authors also performed ethenolysis of methyl oleate for comparison with recycled cooking oil methyl esters and canola methyl esters. They realized the highest turnover frequency value for the ethenolysis of methyl oleate.
The outcomes of their study demonstrated that microwave-assisted conversion of renewable oils together with their fatty acid derivatives is a promising as well as a rapid process for the synthesis of a number of materials for use in chemical industries.
Reference
Aman Ullah and Muhammad Arshad. Remarkably Efficient Microwave-Assisted Cross-Metathesis of Lipids under Solvent-Free Conditions. ChemSusChem 2017, 10, 2167 – 2174.
Go To ChemSusChem Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.
Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.


