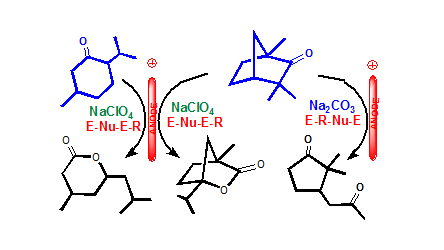
Significance Statement
Researchers led by Professors Belen Batanero and Fructuoso Barba from Department of Organic Chemistry at University of Alcala in Spain studied how some cycloalkanones can be anodically oxidized into lactones and other interesting derivatives when placed under hydro-alcoholic medium. The authors made use of an electrochemical process to carry out their research. This methodology converts terpenoid ketones Fenchone and Menthone into the corresponding lactones when electrolysis is performed using a neutral electrolyte (NaClO4). When alkaline Na2CO3 was employed as electrolyte in the Fenchone oxidation, the fragrance-analogue 2,2-dimethyl-3-(2-oxopropyl) cyclopentanone was obtained in good yield.
The electrochemical process of oxidizing cycloalkanones into lactones used by Batanero et al. (2016) can not be performed by using conventional methods. The research team was able to achieve 75% yield of the Magnolione analog when using alkaline electrolyte. This result supports, once more, the advantages of environmentally-friendly electrochemical technology in the synthesis of highly demanded organic compounds.
E:Electrochem., R: Rearranagement, Nu: Nucelophilic attack.

About the author
Fructuoso Barba is a full Professor at the Organic Chemistry Department of the University of Alcalá (Madrid) Spain. He received his PhD in 1970 at the University of Murcia (Spain) where he returned after one year post-doctoral stay in the group of Prof. Lennart Eberson at the Kemicentrum of the Lund University (Sweden), where he was research fellow. From 1966 to 1986 he was Assistant Professor and Titular Professor at the University of Murcia. In 1986 he became full Professor at the Alcalá de Henares University (Madrid, Spain) where he is now Emeritus Professor.
Prof. Barba has published about 150 papers in Organic Chemistry and Organic Electrochemistry and has made multiple contributions with books, collections of chemistry and oral lectures in Meetings and Symposia on these areas. He has directed many doctoral thesis (PhD) and research projects, being a prominent chemist in Spain. Currently is one of the more active Emeritus researcher, and is the spirit of the spanish Organic Electrochemistry.

About the author
Belén Batanero is a Professor at the University of Alcalá. She received her PhD in 1994 from the University of Alcalá (Madrid) Spain. From 1995 to 1997 she was granted with a EU-Human Capital and Mobility Program followed by a Spanish Ministry fellow, to do post-doctoral research in the group of Prof. Hans J. Schäfer at the Organisch-Chemisches Institüt of the Münster University (Germany). Then she joined again the Organic Chemistry Department of the University of Alcalá (three years MEC-reincorporation contract and subsequently five years as a Ramon y Cajal researcher until 2007), where she get a permanent position as Titular Professor.
Her research interests is focused on Organic Electrosynthesis, mechanism proposals in organic reactions, intermediates in synthetic procedures or the formation of new heterocycles at the electrode, for being applied in medicine and new materials and technology. She has published more than 60 international articles, in specialized and high impact journals, has been coauthor of some book chapters, directed three PhDs and invited speaker in many discussions and conferences in Organic chemistry and Electrochemistry. Since 2013 she is leader of the research group Organic Electrosynthesis at the University of Alcalá (Madrid) Spain.
Journal Reference
Belen Batanero, , Javier Recio, Fructuoso Barba. One-pot Anodic Lactonization of Fenchone and Menthone and Electrosynthesis of a new Magnolione Analogue. Electrochemistry Communications, Volume 66, 2016, Pages 29–33.
Department of Organic Chemistry, University of Alcala, 28871, Alcala de Henares, Madrid, Spain.
Abstract
The terpenoid cycloalkanones Fenchone and Menthone have been oxidized at a platinum anode under neutral and alkaline electrolyte conditions. When NaClO4 was used as electrolyte, Fenchone (1a) afforded 1-isopropyl-4-methyl-2-oxa-bicyclo[2.2.1]heptan-3-one, and Menthone (1b) provided the stable lactone 6-isobutyl-4-methyl-tetrahydropyran-2-one, both in good yield. When using Na2CO3 as electrolyte, the oxidation of 1a gave the fragrance-analogue 2,2-dimethyl-3-(2-oxopropyl) cyclopentanone in only one-pot. Mechanism proposals are presented.
Go To Electrochemistry Communications

 Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.
Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.



