At Four Times Faster Rate in Propylene Carbonate-Based Nonflammable Liquid Electrolyte
Significance
Global shift towards green energy driven-systems has spurred much research focusing on the development of more reliable batteries. As such, there has been a surge in demand for high energy density, long lifetime, high safety and low-cost lithium-ion batteries. More so, with a widening application spectrum (ranging from basic house hold equipment to electric vehicles) lithium-ion batteries continue to attract more interest amongst scientist who anticipate their predominance as commercial power sources. In essence, modern lithium ion batteries are based on the battery chemistry of lithium metal oxide cathode and graphite anode and their interfacial interaction with organic liquid electrolyte that is composed of ethylene carbonate (EC), linear carbonate(s) and additive(s). Literature has it that EC is the preferred cyclic carbonate due to its high ionic conductivity, reasonable electrochemical redox stability, compatible interfaces to both graphite anode and lithium metal oxide cathode and the formation of a surface protective solid-electrolyte interphase (SEI) layer at both anode and cathode. However, high melting point (36.4 °C) of EC imposes the limited operation temperature range of lithium ion batteries above room temperature, which requires the installation of a thermal management system on the battery package of EV and stationary energy storage system (ESS). More importantly, highly flammable EC-based commercial electrolyte is the main cause for battery fire, fire propagation and explosion that are serious threats to the safety of users of EV and ESS.
Recent advances have revealed that Propylene carbonate (PC) offers practical benefit over EC given its lower melting point and higher anodic stability. Nonetheless, PC has been excluded in commercial lithium-ion batteries due to its interfacial incompatibility with graphite anode. In fact, combination of graphite anode with PC-based electrolyte in the absence of any functional electrolyte additive has thus been recognized to be the worst choice. Therefore, there is a need to resolve the PC-graphite combination, probably through the development of a better working and and safer electrolyte beyond conventional one, to allow for wider range of application. In this regard, Chungnam National University researchers led by Professor Seung-Wan Song invented in 2017 and proposed for the first time PC and fluorinated linear carbonates-based nonflammable organic liquid electrolytes [1,2] that can replace the existing flammable electrolytes. In recent study, Chungnam National University researchers: Jisoo Han, Gyeong Jun Chung and led by Professor Seung-Wan Song, reported aiming to clarify on how PC-based nonflammable electrolyte interplays with graphite anode and thus how SEI forms. Their work is currently published in the research journal, ChemSusChem.
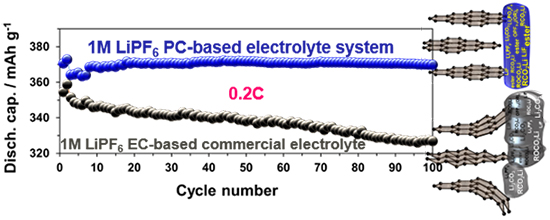
In their approach, the research team provided experimental evidence for the formation and composition of a stable SEI layer on an artificial graphite anode in the PC/DFDEC-based novel electrolyte system without and with 1 wt% FEC additive and the achievement of closely theoretical capacity of graphite under unusually high rate of 0.2 C (charge in 5 hrs), which is generally attainable only at a very low rate below 0.05 C (charge in 20 hrs) in commercial electrolyte.
The authors reported a breakthrough beyond the conventional perception not only of graphite anodes, for which theoretical capacity is attainable only at very low charge-discharge rates (e. g. <0.05 C), but also of PC, which causes the exfoliation of graphene layers and a sudden performance failure of graphite anode and battery death. Moreover, systematic analysis on surface chemistry and structural change of graphite anode revealed that in PC-based nonflammable electrolyte, early produced less resistive SEI layer contained organics-rich with ester, alkyl carbonate salt, organic phosphorus fluorides in the two formation cycles (F1, F2), which continued up to the 100th cycle at 0.2 C, saying it a robust SEI layer.
In summary, the study demonstrated that a graphite anode produces close to the theoretical capacity and unprecedentedly high performance in PC- and fluorinated linear carbonate-based nonflammable electrolyte, even at an unusually high rate of 0.2 C. Remarkably, the strong effects of anode-electrolyte interfacial reaction pathways and the SEI stabilization preventing the exfoliation of graphene sheets and structural degradation of graphite, in promoting the electrochemical reaction reversibility and approaching the theoretical capacity were demonstrated. In a statement to Advances in Engineering, Professor Seung-Wan Song explained the novel combination of graphite with PC-based nonflammable liquid electrolyte ultimately paves the way to their use in high-performance, high-safety and faster charged Li-ion batteries.
References
[1] Hieu Quang Pham, Hee-Yeol Lee, Eui-Hyung Hwang, Young-Gil Kwon, Seung-Wan Song, Non-flammable Organic Liquid Electrolyte for High-safety and High-energy Density Li-ion Batteries. Journal of Power Sources 2018: volume 404, page 13-19
[2] Hieu Quang Pham, Eui-Hyung Hwang, Young-Gil Kwon, Seung-Wan Song, Approaching the Maximum Capacity of Nickel-rich LiNi0.8Co0.1Mn0.1O2 Cathodes by Charging to High-voltage in a Non-flammable Electrolyte of Propylene Carbonate and Fluorinated Linear Carbonates. Chemistry Communications 2019: volume 55, page 1256-1258
[3] Jisoo Han, Gyeong Jun Chung, Seung-Wan Song. Robust Solid-Electrolyte Interphase Enables Near Theoretical Capacity of Graphite Battery Anode at 0.2 C in Propylene Carbonate-Based Electrolyte. ChemSusChem 2020: volume 13, page 5497 –5506
 Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.
Advances in Engineering Advances in Engineering features breaking research judged by Advances in Engineering advisory team to be of key importance in the Engineering field. Papers are selected from over 10,000 published each week from most peer reviewed journals.


